ScRipt | Pharmacy Update
December 2023
What's New
Welcome to the new ScRipt Newsletter!
We have updated and upgraded the ScRipt newsletter to make it easier to read and share. You’ll notice these new features:
- Cleaner format with easier-to-read fonts. We’ve made the font type larger and easier to read, with consistent formatting, which did not always occur with the previous platform.
- Easy-to-print function. Simply click the red button on the right-hand side.
- Spotlight sidebar, giving readers another way to see what’s coming up or what they should be aware of.
Our goal is to continue to improve and deliver the content you need, so we welcome your feedback. Please send us your comments to PharmacyCommunications@independenthealth.com.
Clinical Corner
GSK discontinuing Flovent HFA and Diskus
Independent Health will add generic versions of the products to our formularies to maintain care.
In June, GlaxoSmithKline (GSK) notified the U.S. Food and Drug Administration (FDA) that they will discontinue branded Flovent HFA (all strengths) and Flovent Diskus (all strengths) on December 31, 2023.
To ensure that patients still have access to fluticasone propionate, GSK will continue to manufacture an authorized generic of Flovent HFA and plans to launch an authorized generic of Flovent Diskus in late 2023.
In response to these discontinuations, Independent Health will add the authorized generics of these products to our formularies for all plans as noted below:
- Fluticasone propionate inhalation aerosol (generic Flovent HFA) will be added to our formularies effective November 1, 2023.
- Fluticasone propionate inhalation powder (generic Flovent Diskus) will be on our formularies effective January 1, 2024 or as soon as available.
It is important for patients to continue to take their medication. Flovent prescriptions can be automatically substituted for the generic for most patients. For patient’s with a “DAW” Flovent prescription, a new prescription is needed.
For the most up to date information on covered products, please refer to the Drugs Covered page on our website.
NYSDOH advises RSV vaccinations during 32 through 36 weeks of pregnancy
New statewide standing order allows pharmacies to administer the immunization.
The New York State Department of Health (DOH) recently shared updated information from the Centers for Disease Control and Prevention (CDC) regarding the RSV Immunization, BeyfortusTM (nirsevimab). The CDC advised that there is limited availability of nirsevimab, the long-acting monoclonal antibody immunization recommended for the prevention of RSV-associated lower respiratory tract infection in infants. You can read the full CDC advisory here.
In response to the anticipated nirsevimab shortage, DOH advises administering a single dose of Pfizer’s bivalent RSVpreF vaccine (Abrysvo) during weeks 32 through 36 of pregnancy. Administering the vaccine to this population of pregnant people will provide newborns with protection from RSV-associated lower respiratory tract infection, avoiding the need for most newborns to receive nirsevimab.
What pharmacies can do
A new statewide standing order allows pharmacies to administer the Abrysvo to pregnant people during weeks 32 through 36 of their pregnancy. Pharmacies with Medical Directors (typically chains) may have their own standing order. Pharmacies not using the statewide standing order may not be able to administer Abrysvo. To avoid any delay, providers were also advised to give patients a patient-specific prescription.
The full CDC recommendations for maternal RSV vaccinations are posted here.
For questions on RSV vaccine coverage, please call Independent Health’s Pharmacy Services Department at (716) 631-2934 or 1-800-247-1466, Monday through Friday from 8 a.m. to 5 p.m.
Operational Matters
Diagnosis code requirement for GLP-1s indicated for type-2 diabetes
Ongoing GLP-1 review to ensure proper treatment and preserve access.
Independent Health regularly reviews claims utilization as part of our audit process. In early 2023, a review of glucagon-like peptide-1 (GLP-1) receptor agonists for the treatment of type-2 diabetes showed a significant increase in members filling these agents without an FDA approved indication.
To ensure appropriate medication use and preserve access for patients requiring GLP-1s for glucose control, Independent Health implemented the process outlined below.
Effective July 2023, submission of a valid diagnosis code is required for coverage of the following GLP-1 agonists: Ozempic, Mounjaro, Trulicity, Rybelsus, Victoza
- Diagnosis codes were proactively added for members identified with type-2 diabetes through medical claims. Independent Health continues to update these codes monthly.
- Pharmacies are required to enter a type-2 diagnosis code for members who cannot be proactively verified. Note: "E11" diagnosis codes should only be submitted when documented and verified with the prescriber.
- Independent Health will recover on claims inappropriately coded. If selected for audit, documents from your pharmacy records will be requested to confirm the diagnosis as submitted on the claim.
Effective January 1, 2024, Independent Health will require Step Therapy through drugs that treat type 2 diabetes for all Medicare Advantage members.
- Medicare members currently taking the medication will have the ability to continue treatment.
- New starts must go through the step therapy requirements. If a new start does not meet the step, a coverage determination will need to be submitted.
- As a reminder, Centers for Medicare & Medicaid Services (CMS) excludes all medications used exclusively for weight loss and does not allow coverage for drugs like Zepbound, Wegovy, and Saxenda. Glucagon-like peptide-1 (GLP-1) receptor agonists not indicated for weight loss, like Ozempic and Mounjaro, should not be prescribed for this purpose alone.
It is important to confirm the diagnosis entered with each medication fill and remove any codes that have not been verified by the prescriber. Inappropriate diagnosis codes submitted on the original claim can carry over to subsequent refills on the same prescription.
Please call our pharmacy help desk number at (716) 631-2927 or (800) 993-9898 if you have questions.
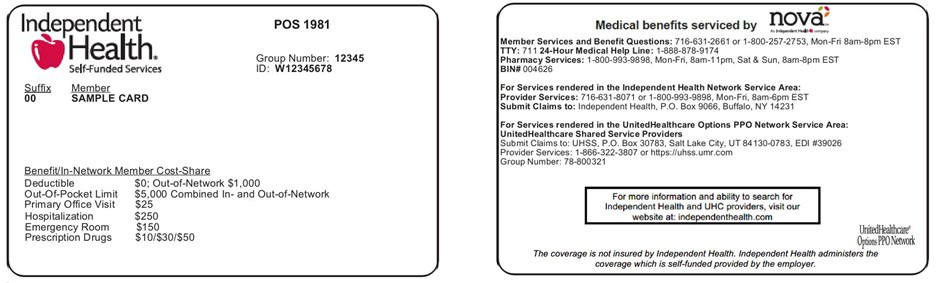
New Member ID Numbers
Self-funded and commercial members will be assigned new ID numbers that begin with W.
Independent Health is issuing new ID cards with new ID numbers to commercial and self-funded members upon their plans’ renewal dates.
What’s different
The new ID numbers for commercial and self-funded members will begin with “W.” (The ID numbers for Medicare and State program members will not change).
The new ID cards will also include a logo of a national network which will serve as the travel network for members who live locally, and as the network for members who live outside of Independent Health’s traditional Western New York service area.
How to prepare
It is important to ask patients if they have new ID cards and to use their new Member ID numbers. While this should have no effect on your billing because the new ID number will track to the member’s current ID number, we ask providers to begin using the new ID numbers as members receive their new cards.
Timing
A limited number of self-funded members have already received their ID cards before December 1.
Starting in December, commercial group members with a January 1 renewal date will begin receiving their new ID cards in December. Members may begin using their new ID cards as soon as they receive them.
ID Card Samples that will have the new Independent Health ID number.
Commercial, fully insured members

Self-funded members
Formulary and Policy Changes
Fourth quarter changes are available for review in PDF. We encourage you to open and download them, as they contain important information and updates:
Policy changes for the Fourth Quarter of 2023 are summarized here.
Formulary changes for the Fourth Quarter of 2023 are summarized here.
View the most up to date versions of Independent Health’s policies when logged in to our provider portal.
Independent Health's drug formularies
Access Independent Health's drug formularies here.
Spotlight
Top Takeaways this Month
Update your digital contact information: Having up to date contact information is crucial. Email is our primary form of contact. Please reach out to PharmacyCommunications@independenthealth.com to provide a current email address so we can continue to share pertinent info, like regulatory changes, planned system downtime, and more.